Electrochemistry¶
Galvanic (Voltaic) Cells¶
- class chemlib.electrochemistry.Galvanic_Cell(self, electrode1: str, electrode2: str)¶
- Parameters
(str) (electrode2) – The elemental composition of one of the electrodes of the galvanic cell.
(str) – The elemental composition of the other electrode of the galvanic cell.
- Raises
NotImplementedError – If either of the electrodes is invalid or its reduction potential is unknown.
Make a Galvanic Cell with Lead and Zinc electrodes:
>>> from chemlib import Galvanic_Cell
>>> g = Galvanic_Cell("Pb", "Zn")
>>>
- chemlib.electrochemistry.Galvanic_Cell.properties: dict¶
A dictionary of the cell’s properties:
>>> g.properties
{'Cell': 'Zn | Zn2+ || Pb2+ | Pb', 'Anode': 'Zn', 'Cathode': 'Pb', 'Cell Potential': 0.63}
>>>
- chemlib.electrochemistry.Galvanic_Cell.cell_potential: float¶
Access the cell potential of the galvanic cell:
>>> g.cell_potential
0.63
>>> g.E0
0.63
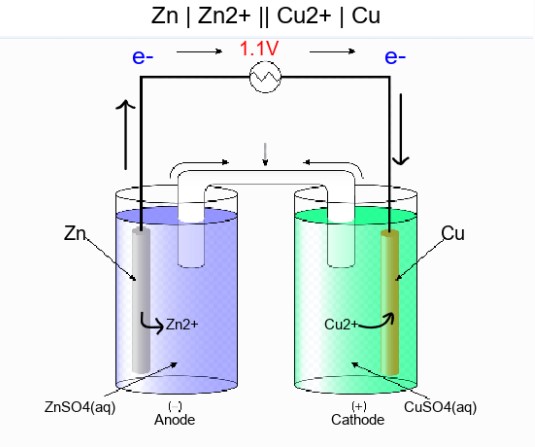
- chemlib.electrochemistry.Galvanic_Cell.diagram: PIL.Image¶
The diagram of the galvanic cell is a PIL.Image object.
To generate diagram:
>>> g.draw()
To save (as png file):
>>> g.diagram.save("filename.png")
>>>
Electrolysis¶
- chemlib.electrochemistry.electrolysis(element: str, n: int, **kwargs) dict:¶
- Parameters
(str) (element) – The symbol of a chemical element.
(int) (n) – The moles of electrons transferred.
kwargs – Provide two of the values from amps, seconds, and grams.
- Raises
TypeError – If not only 2 of the parameters in kwargs are specified.
Example: Copper metal is purified by electrolysis. How much copper metal (in grams) could be produced from copper (ii) oxide by applying a current of 10.0 amps at the appropriate negative potential for 12.0 hours?
>>> from chemlib import electrolysis
>>> electrolysis('Cu', 2, amps = 10, seconds=12*60*60)
{'element': 'Cu', 'n': 2, 'seconds': 43200, 'amps': 10, 'grams': 142.25979167746283}
>>>
Example: How long would it take to electroplate a flute with 28.3 g of silver at a constant current of 2.0 amps using AgNO3?
>>> from chemlib import electrolysis
>>> electrolysis("Ag", 2, amps = 2, grams = 28.3)
{'element': 'Ag', 'n': 2, 'seconds': 25313.582341380206, 'amps': 2, 'grams': 28.3}
>>>
Example: How much current was used to produce 805 grams of Aluminum metal from Al2O3 in 24 hours?
>>> from chemlib import electrolysis
>>> electrolysis("Al", 3, grams = 805, seconds = 24*60*60)
{'element': 'Al', 'n': 3, 'seconds': 86400, 'amps': 99.95144010616133, 'grams': 805}
>>>